If you're not happy, return this item for a full refund.


eXciteOSA Sleep Apnea Therapy Device Starter Pack (BACKORDER)
-
30-DAY MONEY BACK GUARANTEE
If you're not happy, return this item for a full refund.30-DAY-NIGHT RISK FREE TRIAL -
BUY NOW, PAY LATER: 0% FINANCING
BUY NOW, PAY LATER
Description
| 30-DAY MONEY BACK GUARANTEE |
FDA AUTHORIZED PRESCRIPTION SLEEP THERAPY
eXciteOSA is the only product of its kind authorized for the treatment of OSA; and it is available by prescription only. A standard CPAP or BiPAP prescription is not valid for the purchase of eXciteOSA. eXciteOSA prescriptions must state "eXciteOSA" or something similar. Click here to download a eXciteOSA Prescription Form to bring to your doctor.
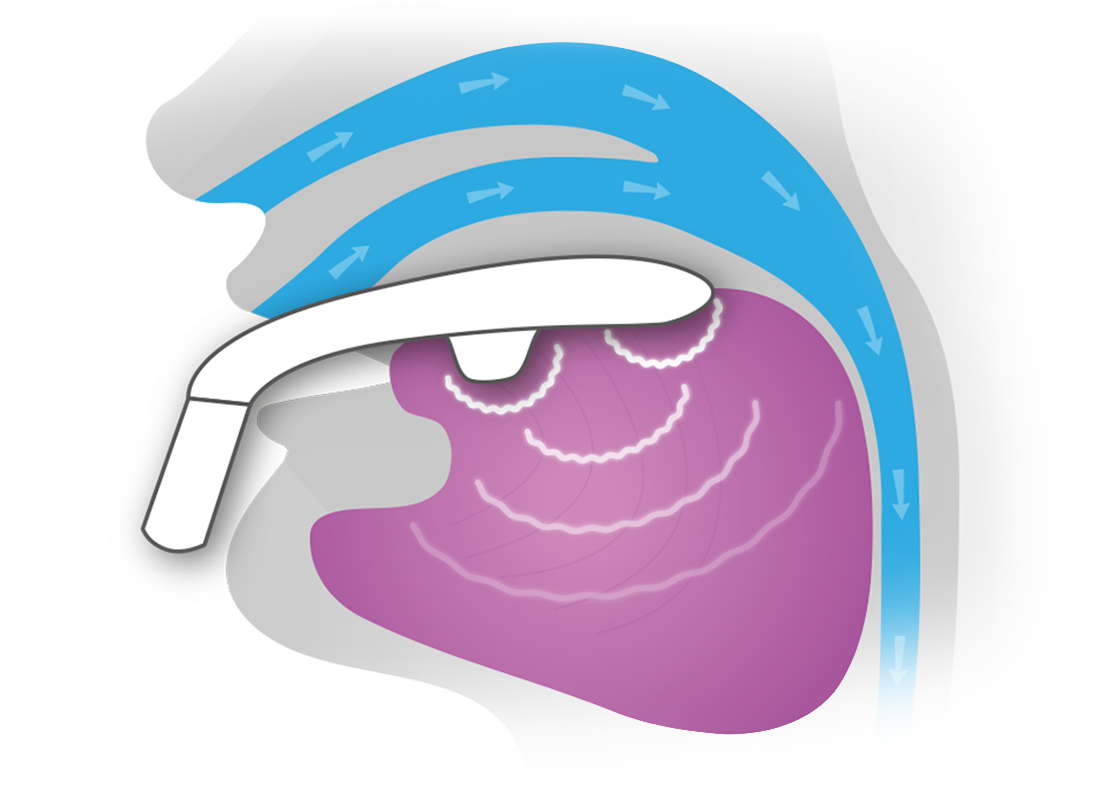
The eXciteOSA Daytime Therapy Device (formerly known as Snoozeal) uses safe, mild electrical currents to exercise and strengthen your tongue muscles and help reduce snoring and treat mild Obstructive Sleep Apnea. Use eXciteOSA whenever it's convenient for 20 minutes a day, every day for 6 weeks. After that, use eXciteOSA once a week to keep your muscles toned. You'll be able to monitor your therapy progress, track results, and share data with your doctor easily using the free eXciteOSA App.
|
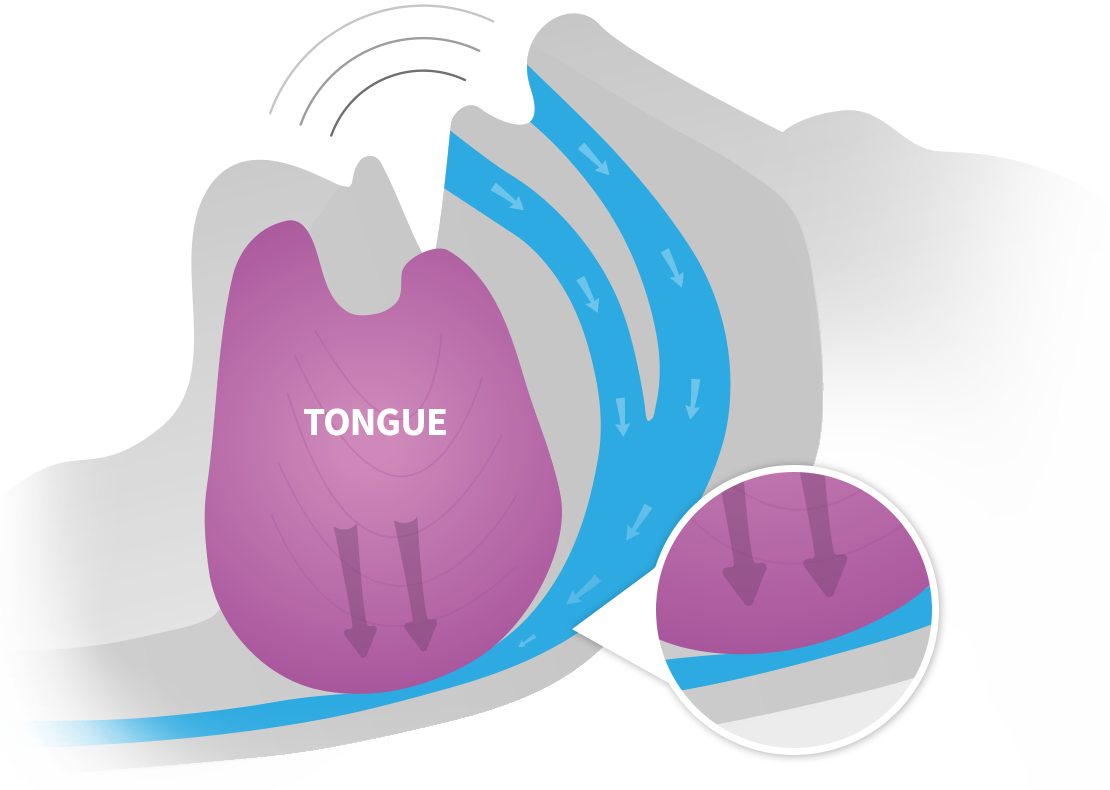
WHAT CAUSES SNORING? |
|
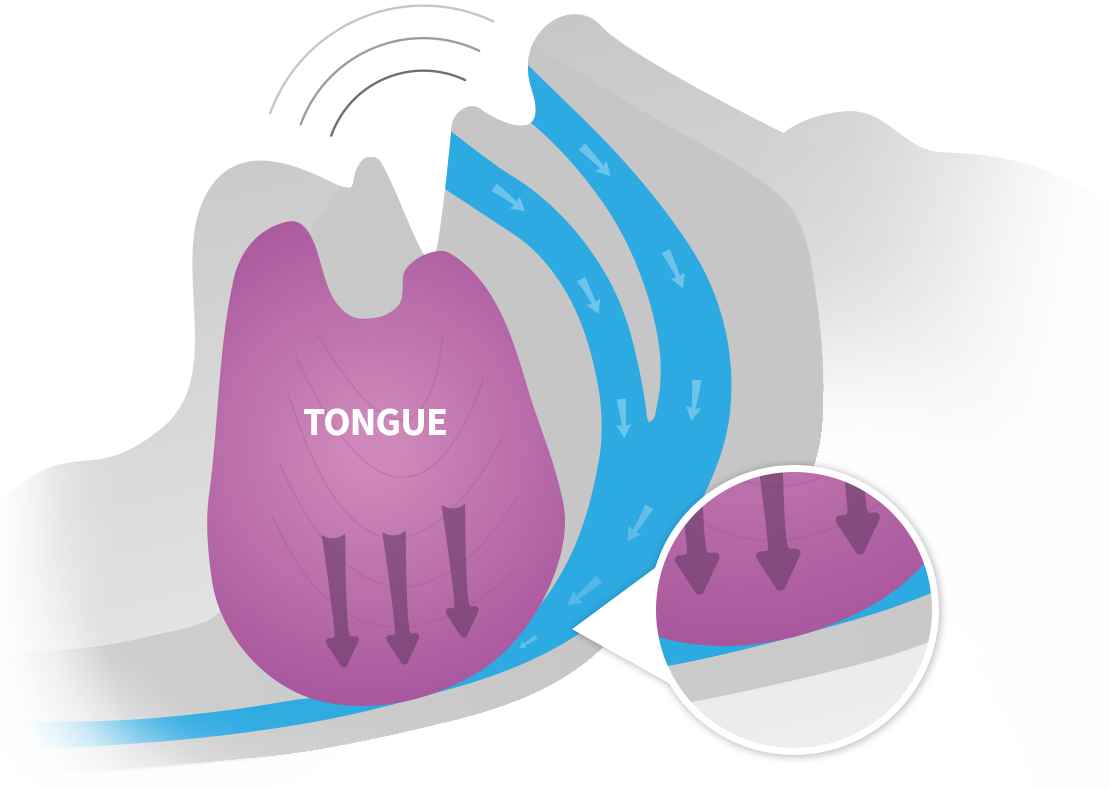
LOUD SNORING CAN BE A SIGN OF SLEEP APNEA |
|
EXCITEOSA TARGETS THE ROOT CAUSE OF SNORING |
|
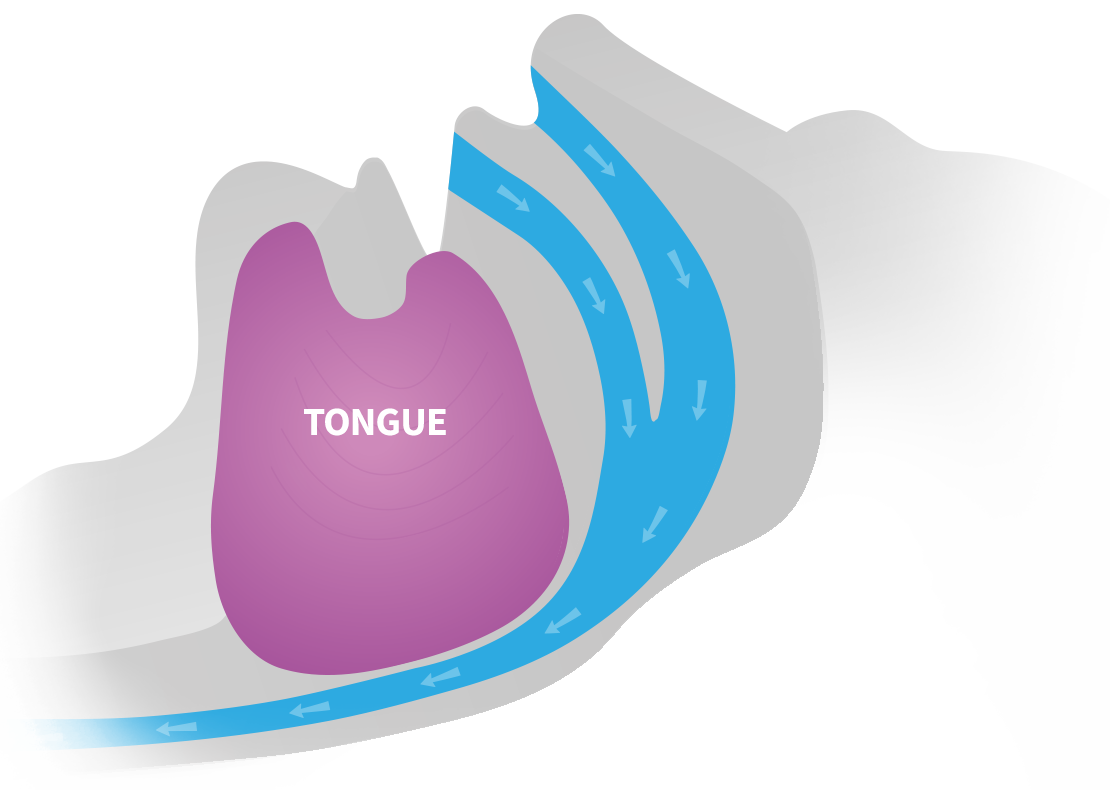
BETTER QUALITY SLEEP & A HEALTHIER LIFE |
|
CONTROL & TRACK THERAPY PROGRESS WITH THE EXCITEOSA APP |
UPDATED WITH LED INDICATOR LIGHT & EXTENDER CABLE
The eXciteOSA Device Starter Pack now comes with an extender cable and features an LED indicator light on the control unit.
The LED LIGHT on eXciteOSA's Control Unit has several functions. It will flash blue and yellow when your device needs to be recharged before the next therapy session. During charging it will blink blue; and when fully charge the LED light will shine continously blue. If the LED light on your eXciteOSA Control Unit flashes red and yellow while charging it means that moisture has been detected in the USB-C Connector. If this happens disconnect the control unit and lit it dry for a minimum of 4 hours before attempting to charge again.
The EXTENDER CABLE can be placed between the eXciteOSA Mouthpiece and Control Unit to ensure the control unit does not get wet due to excess moisture during use. It is included in the eXciteOSA Starter Kit for optional use based on the user's preference.
^literature~- eXciteOSA Therapy Product Brochure (PDF)
- eXciteOSA Therapy Quick Start Guide (PDF)
- eXciteOSA Therapy What to Expect (PDF)
- eXciteOSA Understanding Level Settings (PDF)
- eXciteOSA Instructions For Use (PDF)
- eXciteOSA Clinical Brochure (PDF)
- eXciteOSA Clinical FAQs (PDF)
- eXciteOSA White Paper (PDF)
Product Information
- Manufacturer Signifier Medical Technologies
- In the Package Control Unit, Mouthpiece, Extender Cable (for Optional Use), USB Charging Cable, Printed User Manual, Manufacturer's Warranty
- Part Number(s) 13010
- HCPCS Code K1028
- GTIN / UPC Code 005060627480048
- Warranty 1 Year - Review manufacturer's documentation for full warranty details.
- Intended Use eXciteOSA is indicated for use to reduce snoring for patients that are 18 years or older. Contraindications: Do not use eXciteOSA if you are pregnant or may be pregnant; you have a pacemaker or implanted electrodes; you have temporary or permanent implants, dental braces or jewellery in your mouth; you are suffering from mouth ulceration; you have or are suspected of having sleep apnea with an AHI of greater than 15. Safety and effectiveness in the above conditions has not been established. Read all instructions before use.
- Prescription Item Prescription required for the purchase of this item.
- Country United Kingdom (Typical Origin)